Abstract
Introduction: High dose methotrexate (HD-MTX) treatment needs close monitoring, forced diuresis using intravenous hydration to ensure rapid drug clearance, and leucovorin rescue. Delayed clearance can result in serious adverse events such as severe mucositis and pancytopenia. Most institutions keep patients hospitalized until complete drug clearance, which can take up to 4-5 days on average (Binder, A et al.). Patients who exhibit normal drug clearance kinetics exhibit no increase in toxicity.
Method: We developed modified protocol at Regions Hospital where patients can be discharged if their initial post-infusion methotrexate drug levels demonstrate normal clearance kinetics [< 5 mM (micromoles/L) at 24 hours or < 1 mM at 48 hours], they are able to maintain adequate oral hydration and have no evidence of renal or hepatic dysfunction. Patients are prescribed oral leucovorin at discharge to complete 7 days of treatment. Many institutions use a protocol that involves serial methotrexate drug level monitoring each day until the level is below 0.1-0.05 mM. We conducted a retrospective chart review to determine if the modified protocol is comparable to the commonly used standard protocol in terms of safety. We summarized patient- and cycle-specific characteristics and assessed the incidence of renal, hepatic, gastrointestinal, and hematologic toxicities among patients treated with HD-MTX (defined as ≥ 1000 mg/m2).
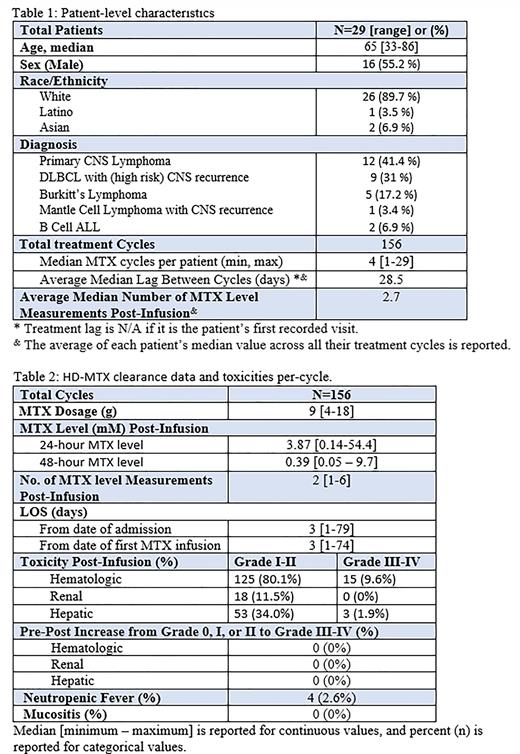
Results: We retrospectively reviewed medical records of 53 patients who received intravenous methotrexate in our institution between 2015-2019. Only 29 patients had received HD-MTX (defined as ≥ 1000 mg/m2). Median age was 65 years [33-86], 55.2% (n=16) were male. HD MTX was given for B-cell lymphoma with high risk for CNS recurrence in 15 patients (51.7%), primary CNS lymphoma in 12 patients (41.4%) and acute lymphocytic lymphoma in 2 patients (6.9%). Median MTX dosage was 9 g [4-18]. Median 24-hour MTX-level was 3.78 mM and 48- hour MTX-level was 0.39 mM. (Tables 1-2).
Of the total number of 156 cycles, grade III or more of hematological, hepatic, and renal toxicities were reported in (9.6%, 1.3% and 0%) respectively. There was no increase from low grade (0, I, II) to high grade (III, IV) toxicities. Mucositis was not reported after any of these cycles; however, febrile neutropenia occurred after 4 cycles (2.4%). Overall, there was no increase in toxicity compared to toxicity of historical protocol (Stoller RG. et al.). Length of hospital stay (LOS) was 3 days [1-79] comparing with 4-5 days to what reported historically. (Table 2).
Conclusion: Our date suggests that patients who received high dose methotrexate can be safely discharged early if they exhibit normal drug clearance kinetics with in the first 48 hours after infusion, maintain normal renal function and continue oral hydration and leucovorin as outpatient. Adopting this protocol may have an economic benefit by shortening the length of hospitalization by 1-2 days.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal